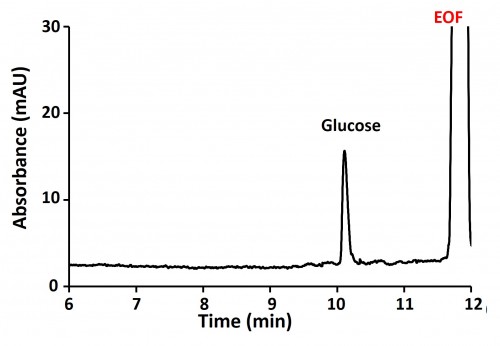
Glucose is the main component of pediatric Total Parenteral bags.
The control of Glucose content in pharmaceutical preparations is an important step before delivering bags to patients. This application describes the use of the Wyn-CE Capillary Electrophoresis system with a UV detection for the determination of Glucose in TPN preparation.
L'avis de l'expert : Glucose analysis is a complementary application to cation analysis for the quality control of total parenteral nutrition formulation.
Réactifs et conditions d'analyse
Tampon : Sugar WynSep buffer, pH 12.2
Capillaire : bare-fused silica, L = 70 cm, ID = 75 μm
Injection : hydrodynamic, 50 mbar, 5 s
Tension : -25 kV
Détection : Indirect UV, 254 nm
Température : 25 °C
Spécifications